Practice Essentials
Necrotizing fasciitis is a rapidly progressive inflammatory infection of the fascia, with secondary necrosis of the subcutaneous tissues. The speed of spread is directly proportional to the thickness of the subcutaneous layer. Necrotizing fasciitis moves along the fascial plane. [1, 2, 3]
Necrotizing fasciitis has also been referred to as hemolytic streptococcal gangrene, Meleney ulcer, acute dermal gangrene, hospital gangrene, suppurative fasciitis, and synergistic necrotizing cellulitis. Fournier gangrene is a form of necrotizing fasciitis that is localized to the scrotum and perineal area.
Necrotizing fasciitis may occur as a complication of a variety of surgical procedures or medical conditions, including cardiac catheterization, [4] vein sclerotherapy, [5] and diagnostic laparoscopy, [6] among others. [7, 8, 9, 10, 11, 12, 13] It may also be idiopathic, as in scrotal or penile necrotizing fasciitis.
The causative bacteria may be aerobic, anaerobic, or mixed flora. [14] A few distinct necrotizing fasciitis syndromes should be recognized. The 3 most important are as follows:
-
Type I, or polymicrobial
-
Type II, or group A streptococcal
-
Type III gas gangrene, or clostridial myonecrosis
A variant of necrotizing fasciitis type I is saltwater necrotizing fasciitis, in which an apparently minor skin wound is contaminated with saltwater containing a Vibrio species.
Because of the presence of gas-forming organisms, subcutaneous air is classically described in necrotizing fasciitis. This may be seen only on radiographs or not at all.
The frequency of necrotizing fasciitis has been on the rise because of an increase in immunocompromised patients with diabetes mellitus, cancer, alcoholism, vascular insufficiencies, organ transplants, human immunodeficiency virus (HIV) infection, or neutropenia.
Since 1883, more than 500 cases of necrotizing fasciitis have been reported in the literature. There may be an increased incidence in African and Asian countries; however, because of the lack of recorded cases, the true incidence is not known.
The mean age of a patient with necrotizing fasciitis is 38-44 years. This disease rarely occurs in children. Pediatric cases have been reported from resource-poor nations where poor hygiene is prevalent. The male-to-female ratio is 2-3:1.
These infections can be difficult to recognize in their early stages, but they rapidly progress. (see Clinical and Workup). They require aggressive treatment to combat the associated high morbidity and mortality (see Treatment).
Images of necrotizing fasciitis are shown below.
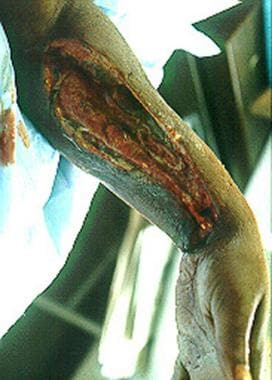 Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
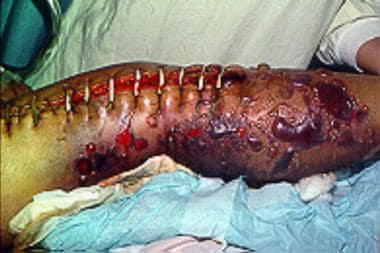 Left lower extremity in a 56-year-old patient with alcoholism who was found comatose after binge drinking. Surgical drainage was performed to treat the pyomyositis-related, large, non–foul-smelling (sweetish) bullae. Gram staining showed the presence of gram-positive rods. Cultures revealed Clostridium perfringens. The diagnosis was clostridial myonecrosis.
Left lower extremity in a 56-year-old patient with alcoholism who was found comatose after binge drinking. Surgical drainage was performed to treat the pyomyositis-related, large, non–foul-smelling (sweetish) bullae. Gram staining showed the presence of gram-positive rods. Cultures revealed Clostridium perfringens. The diagnosis was clostridial myonecrosis.
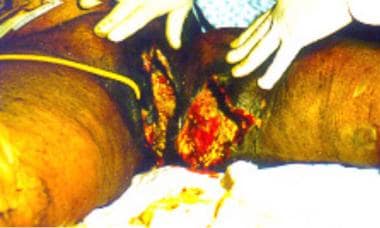 Sixty-year-old woman who had undergone postvaginal hysterectomy and repair of a rectal prolapse has a massive perineal ulceration with foul-smelling discharge. Cultures revealed Escherichia coli and Bacteroides fragilis. The diagnosis was perineal gangrene.
Sixty-year-old woman who had undergone postvaginal hysterectomy and repair of a rectal prolapse has a massive perineal ulceration with foul-smelling discharge. Cultures revealed Escherichia coli and Bacteroides fragilis. The diagnosis was perineal gangrene.
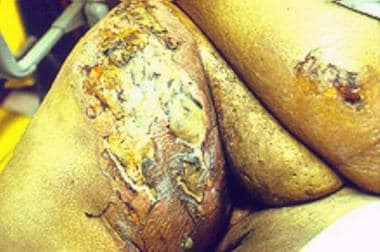 Necrotizing fasciitis at a possible site of insulin injection in the left upper part of the thigh in a 50-year-old obese woman with diabetes.
Necrotizing fasciitis at a possible site of insulin injection in the left upper part of the thigh in a 50-year-old obese woman with diabetes.
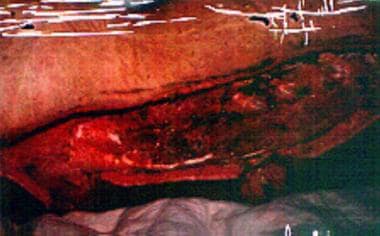 Necrotizing fascitis of entire thoracolumbar posterior area in 20-year-old patient with chronic myelogenous leukemia and neutropenia (WBC count, 680/uL). Cultures revealed gram-negative Pseudomonas species and Bacteroides fragilis.
Necrotizing fascitis of entire thoracolumbar posterior area in 20-year-old patient with chronic myelogenous leukemia and neutropenia (WBC count, 680/uL). Cultures revealed gram-negative Pseudomonas species and Bacteroides fragilis.
Historical background
Necrotizing fasciitis was first described by a Confederate Army surgeon, Joseph Jones, during the US Civil War. [15] In 1883, Fournier documented necrotizing fasciitis in the perineal and genital region. [16] Meleney later reported 20 patients he encountered in China in whom necrotizing fasciitis was caused by hemolytic streptococcus. [17] Wilson used the term necrotizing fasciitis without assigning a specific pathologic bacterium that caused the disease. [18]
Smith et al first classified soft tissue infections as either local or spreading. [19] Lewis later further classified soft tissue infections into either necrotizing or non-necrotizing. [20] He further subdivided these infections into either focal or diffuse.
Collective reviews
In 2010, a team of scientists and surgeons from the Legacy Emanuel Shock Trauma Center in Portland, Oregon, wrote a collective review on the diagnosis and treatment of necrotizing fasciitis. These scientists and surgeons found extensive wide debridement of all tissues that can be easily elevated off the fascia with gentle pressure should be undertaken. The use of adjunctive therapies, such as hyperbaric oxygen therapy (HBO), continue to receive considerable attention as a integral parts of the life-saving therapies. [21]
In 2011, Drs Rausch and Foca wrote an exciting report focusing on necrotizing fasciitis in a pediatric patient. At the end of the report, the authors pointed out that all pediatricians must be aware of the possibility of severe infections in pediatric patients with necrotizing fasciitis who had vascular lymphatic malformations. [22]
In 2012, a team of scientists and surgeons from Turkey wrote a comprehensive collective review on necrotizing fasciitis. At the end of their report, they emphasize that early diagnosis of necrotizing fasciitis may be life saving. [23]
At the Imperial College in London, United Kingdom, 5 scholars wrote a comprehensive 5-year review of necrotizing fasciitis. They emphasize that necrotizing fasciitis is a life-threatening disease that is often difficult to diagnose. The authors guide the readers to make the correct diagnosis as soon as possible in order to save lives. [24]
National Necrotizing Fasciitis Foundation (NNFF)
The cofounders of this distinguished foundation are necrotizing fasciitis survivors, Donna Batdorff and Jackie Roemmele. [25] In the years since these 2 women teamed up to be leaders of this society, they have diligently worked in concert with a dedicated volunteer staff to fulfill the foundation’s mission to increase awareness about necrotizing fasciitis. Foundation activities include public speaking and outreach efforts regarding necrotizing fasciitis throughout the world to educate the medical community and to help save lives from this often misdiagnosed, rapidly fatal disease. See National Necrotizing Fasciitis Foundation.
Signs and symptoms of necrotizing fasciitis
The initial necrosis appears as a massive undermining of the skin and subcutaneous layer. If the skin is open, gloved fingers can pass easily between the two layers and may reveal yellowish green necrotic fascia. If the skin is unbroken, a scalpel incision will reveal it.
The normal skin and subcutaneous tissue become loosened from the rapidly spreading deeper necrotic fascia that is a great distance from the initiating wound. Fascial necrosis is typically more advanced than the appearance suggests.
Anesthesia in the involved region may be detected, and it usually is caused by thrombosis of the subcutaneous blood vessels, leading to necrosis of nerve fibers.
Without treatment, secondary involvement of deeper muscle layers may occur, resulting in myositis or myonecrosis.
Usually, the most important signs of necrotizing fasciitis are tissue necrosis, putrid discharge, bullae, severe pain, gas production, rapid burrowing through fascial planes, and lack of classical tissue inflammatory signs.
Workup in necrotizing fasciitis
Laboratory tests, along with appropriate imaging studies, may facilitate the diagnosis of necrotizing fasciitis. [26, 27] Laboratory evaluation should include the following:
-
Complete blood count with differential
-
Serum chemistry studies
-
Arterial blood gas measurement
-
Urinalysis
-
Blood and tissue cultures
Sonography may reveal subcutaneous emphysema spreading along the deep fascia, swelling, and increased echogenicity of the overlying fatty tissue with interlacing fluid collections, allowing for early surgical debridement and parenteral antibiotics. [28]
Computed tomography (CT) scanning can pinpoint the anatomic site of involvement by demonstrating necrosis with asymmetrical fascial thickening and the presence of gas in the tissues. However, note that early on, CT scan findings may be minimal.
Magnetic resonance imaging (MRI) is the preferred technique to detect soft tissue infection because of its unsurpassed soft tissue contrast and sensitivity in detecting soft tissue fluid, its spatial resolution, and its multiplanar capabilities. [29, 30]
In addition, the finger test should be used in the diagnosis of patients who present with necrotizing fasciitis. [31, 32]
Management of necrotizing fasciitis
Although some necrotizing infections may still be susceptible to penicillin, clindamycin is the treatment of choice for necrotizing infections.
Surgery is the primary treatment for necrotizing fasciitis. The authors recommend wide, extensive debridement of all tissues that can be easily elevated off the fascia with gentle pressure.
After the initial debridement, the wound must be carefully examined. Hemodynamic instability is usually present after surgery, and it may cause progressive skin necrosis. After debridement, the patient may return as often as necessary for further surgical debridement. Once all of the affected tissues have been debrided, soft tissue reconstruction can be considered.
Background
Necrotizing fasciitis is a rapidly progressive inflammatory infection of the fascia, with secondary necrosis of the subcutaneous tissues. The speed of spread is directly proportional to the thickness of the subcutaneous layer. Necrotizing fasciitis moves along the fascial plane. [1, 2]
Necrotizing fasciitis has also been referred to as hemolytic streptococcal gangrene, Meleney ulcer, acute dermal gangrene, hospital gangrene, suppurative fasciitis, and synergistic necrotizing cellulitis. Fournier gangrene is a form of necrotizing fasciitis that is localized to the scrotum and perineal area.
Necrotizing fasciitis may occur as a complication of a variety of surgical procedures or medical conditions, including cardiac catheterization, [4] vein sclerotherapy, [5] and diagnostic laparoscopy, [6] among others. [7, 8, 9, 10, 11, 12, 13] It may also be idiopathic, as in scrotal or penile necrotizing fasciitis.
The causative bacteria may be aerobic, anaerobic, or mixed flora. [14] A few distinct necrotizing fasciitis syndromes should be recognized. The 3 most important are as follows:
-
Type I, or polymicrobial
-
Type II, or group A streptococcal
-
Type III gas gangrene, or clostridial myonecrosis
A variant of necrotizing fasciitis type I is saltwater necrotizing fasciitis, in which an apparently minor skin wound is contaminated with saltwater containing a Vibrio species.
Because of the presence of gas-forming organisms, subcutaneous air is classically described in necrotizing fasciitis. This may be seen only on radiographs or not at all.
The frequency of necrotizing fasciitis has been on the rise because of an increase in immunocompromised patients with diabetes mellitus, cancer, alcoholism, vascular insufficiencies, organ transplants, HIV infection, or neutropenia.
According to the Centers for Disease Control and Prevention, an estimated 700-1150 cases of necrotizing fasciitis caused by group A Streptococcus occur annually in the United States. [33]
The mean age of a patient with necrotizing fasciitis is 38-44 years. This disease rarely occurs in children. Pediatric cases have been reported from resource-poor nations where poor hygiene is prevalent. The male-to-female ratio is 2-3:1.
These infections can be difficult to recognize in their early stages, but they rapidly progress. (see Clinical and Workup). They require aggressive treatment to combat the associated high morbidity and mortality (see Treatment).
Images of necrotizing fasciitis are shown below.
 Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
 Left lower extremity in a 56-year-old patient with alcoholism who was found comatose after binge drinking. Surgical drainage was performed to treat the pyomyositis-related, large, non–foul-smelling (sweetish) bullae. Gram staining showed the presence of gram-positive rods. Cultures revealed Clostridium perfringens. The diagnosis was clostridial myonecrosis.
Left lower extremity in a 56-year-old patient with alcoholism who was found comatose after binge drinking. Surgical drainage was performed to treat the pyomyositis-related, large, non–foul-smelling (sweetish) bullae. Gram staining showed the presence of gram-positive rods. Cultures revealed Clostridium perfringens. The diagnosis was clostridial myonecrosis.
 Sixty-year-old woman who had undergone postvaginal hysterectomy and repair of a rectal prolapse has a massive perineal ulceration with foul-smelling discharge. Cultures revealed Escherichia coli and Bacteroides fragilis. The diagnosis was perineal gangrene.
Sixty-year-old woman who had undergone postvaginal hysterectomy and repair of a rectal prolapse has a massive perineal ulceration with foul-smelling discharge. Cultures revealed Escherichia coli and Bacteroides fragilis. The diagnosis was perineal gangrene.
 Necrotizing fasciitis at a possible site of insulin injection in the left upper part of the thigh in a 50-year-old obese woman with diabetes.
Necrotizing fasciitis at a possible site of insulin injection in the left upper part of the thigh in a 50-year-old obese woman with diabetes.
 Necrotizing fascitis of entire thoracolumbar posterior area in 20-year-old patient with chronic myelogenous leukemia and neutropenia (WBC count, 680/uL). Cultures revealed gram-negative Pseudomonas species and Bacteroides fragilis.
Necrotizing fascitis of entire thoracolumbar posterior area in 20-year-old patient with chronic myelogenous leukemia and neutropenia (WBC count, 680/uL). Cultures revealed gram-negative Pseudomonas species and Bacteroides fragilis.
Historical background
Necrotizing fasciitis was first described by a Confederate Army surgeon, Joseph Jones, during the US Civil War. [15] In 1883, Fournier documented necrotizing fasciitis in the perineal and genital region. [16] Meleney later reported 20 patients he encountered in China in whom necrotizing fasciitis was caused by hemolytic streptococcus. [17] Wilson used the term necrotizing fasciitis without assigning a specific pathologic bacterium that caused the disease. [18]
Smith et al first classified soft tissue infections as either local or spreading. [19] Lewis later further classified soft tissue infections into either necrotizing or non-necrotizing. [20] He further subdivided these infections into either focal or diffuse.
Collective reviews
In 2010, a team of scientists and surgeons from the Legacy Emanuel Shock Trauma Center in Portland, Oregon, wrote a collective review on the diagnosis and treatment of necrotizing fasciitis. These scientists and surgeons found extensive wide debridement of all tissues that can be easily elevated off the fascia with gentle pressure should be undertaken. The use of adjunctive therapies, such as hyperbaric oxygen therapy (HBO), continue to receive considerable attention as a integral parts of the life-saving therapies. [21]
In 2011, Drs Rausch and Foca wrote an exciting report focusing on necrotizing fasciitis in a pediatric patient. At the end of the report, the authors pointed out that all pediatricians must be aware of the possibility of severe infections in pediatric patients with necrotizing fasciitis who had vascular lymphatic malformations. [22]
In 2012, a team of scientists and surgeons from Turkey wrote a comprehensive collective review on necrotizing fasciitis. At the end of their report, they emphasize that early diagnosis of necrotizing fasciitis may be life saving. [23]
At the Imperial College in London, United Kingdom, 5 scholars wrote a comprehensive 5-year review of necrotizing fasciitis. They emphasize that necrotizing fasciitis is a life-threatening disease that is often difficult to diagnose. The authors guide the readers to make the correct diagnosis as soon as possible in order to save lives. [24]
National Necrotizing Fasciitis Foundation (NNFF)
The cofounders of this distinguished foundation are necrotizing fasciitis survivors, Donna Batdorff and Jackie Roemmele. [25] In the years since these 2 women have teamed up to be leaders in this society, they have diligently worked in concert with a dedicated volunteer staff to fulfill the foundation’s mission to increase awareness about necrotizing fasciitis. Foundation activities include public speaking and outreach efforts regarding necrotizing fasciitis throughout the world to educate the medical community and to help save lives from this often misdiagnosed, rapidly fatal disease. See National Necrotizing Fasciitis Foundation.
Pathophysiology
Necrotizing fasciitis is characterized by widespread necrosis of the subcutaneous tissue and the fascia. It was once considered an uncommon clinical entity. In the 1990s, the media popularized the idea that this infection was caused by "flesh-eating bacteria." Although the pathogenesis of necrotizing fasciitis is still open to speculation, the rapid and destructive clinical course of necrotizing fasciitis is thought to be due to multibacterial symbiosis and synergy. [15]
Historically, group A beta-hemolytic Streptococcus (GABS) has been identified as a major cause of this infection. This monomicrobial infection is usually associated with an underlying cause, such as diabetes, [34] atherosclerotic vascular disease, or venous insufficiency with edema. GABS usually affects the extremities; approximately two thirds of the GABS infections are located in the lower extremities. [35]
During the last 2 decades, researchers have found that necrotizing fasciitis is usually polymicrobial rather than monomicrobial. [36, 37, 38] Anaerobic bacteria are present in most necrotizing soft-tissue infections, usually in combination with aerobic gram-negative organisms. Anaerobic organisms proliferate in an environment of local tissue hypoxia in those patients with trauma, recent surgery, or medical compromise.
Facultative aerobic organisms grow because polymorphonuclear neutrophils (PMNs) exhibit decreased function under hypoxic wound conditions. This growth further lowers the oxidation/reduction potential, enabling more anaerobic proliferation and, thus, accelerating the disease process.
Carbon dioxide and water are the end products of aerobic metabolism. Hydrogen, nitrogen, hydrogen sulfide, and methane are produced from the combination of aerobic and anaerobic bacteria in a soft tissue infection. These gases, except carbon dioxide, accumulate in tissues because of reduced water solubility.
In necrotizing fasciitis, group A hemolytic streptococci and Staphylococcus aureus, alone or in synergism, are frequently the initiating infecting bacteria. However, other aerobic and anaerobic pathogens may be present, including the following:
-
Bacteroides
-
Clostridium
-
Peptostreptococcus
-
Enterobacteriaceae
-
Coliforms (eg, Escherichia coli)
-
Proteus
-
Pseudomonas
-
Klebsiella
Bacteroides fragilis is usually noted as part of a mixed flora in combination with E coli. B fragilis does not directly cause these infections, but it does play a part in reducing interferon production and the phagocytic capacity of macrophages and PMNs.
A variant synergistic necrotizing cellulitis is considered to be a form of necrotizing fasciitis, but some authorities feel that it is actually a nonclostridial myonecrosis. This condition begins in the same manner as necrotizing fasciitis, but it progresses rapidly to involve wide areas of deeper tissue and muscle at an earlier stage than might be expected. Severe systemic toxicity occurs.
Anaerobic streptococci, occasionally seen in intravenous drug users, cause many forms of nonclostridial myonecrosis (see the image below). Some cases of necrotizing fasciitis can be caused by Vibrio vulnificus. This organism is seen more often in patients with chronic liver dysfunction, and it often follows the consumption of raw seafood. V vulnificus may cause subcutaneous bleeding.
 Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
Organisms spread from the subcutaneous tissue along the superficial and deep fascial planes, presumably facilitated by bacterial enzymes and toxins. This deep infection causes vascular occlusion, ischemia, and tissue necrosis. Superficial nerves are damaged, producing the characteristic localized anesthesia. Septicemia ensues with systemic toxicity.
Important bacterial factors include surface protein expression and toxin production. M-1 and M-3 surface proteins, which increase the adherence of the streptococci to the tissues, also protect the bacteria against phagocytosis by neutrophils.
Streptococcal pyrogenic exotoxins (SPEs) A, B, and C are directly toxic and tend to be produced by strains causing necrotizing fasciitis. These pyrogenic exotoxins, together with streptococcal superantigen (SSA), lead to the release of cytokines and produce clinical signs such as hypotension. The etiological agent may also be a Staphylococcus aureus isolate harboring the enterotoxin gene cluster seg, sei, sem, sen, and seo, but lacking all common toxin genes, including Panton-Valentine leukocidin. [39]
The poor prognosis associated with necrotizing fasciitis has been linked to infection with certain streptococcal strains. Community-acquired methicillin-resistant S aureus (MRSA) has also been associated with necrotizing fasciitis. [40]
Single-nucleotide changes are the most common cause of natural genetic variation among members of the same species. They may alter bacterial virulence; a single-nucleotide mutation in the group A Streptococcus genome was identified that is epidemiologically associated with decreased human necrotizing faciitis. [41]
It was found that wild-type mtsR function is required for group A Streptococcus to cause necrotizing fasciitis in mice and nonhuman primates. It was speculated that a naturally occurring single-nucleotide mutation dramatically alters virulence by dysregulating a multiple gene virulence axis.
Severe myositis accompanying septic necrotizing fasciitis may be caused by a Panton-Valentine leukocidin–positive S aureus strain. [42] Immunostaining may document strong binding of the Panton-Valentine leukocidin toxin to necrotic muscle tissues.
Although necrotizing fasciitis most frequently develops after trauma that compromises skin integrity, it may rarely develop in a healthy person after minor trauma such as an isolated shoulder sprain that occurred without a break in skin barrier. [43]
Etiology
Surgical procedures may cause local tissue injury and bacterial invasion, resulting in necrotizing fasciitis. These procedures include surgery for intraperitoneal infections and drainage of ischiorectal and perianal abscesses. Intramuscular injections and intravenous infusions may lead to necrotizing fasciitis.
Minor insect bites may set the stage for necrotizing infections. Streptococci introduced into the wounds may be prominent initially, but the bacteriologic pattern changes with hypoxia-induced proliferation of anaerobes.
Local ischemia and hypoxia can occur in patients with systemic illnesses (eg, diabetes). Host defenses can be compromised by underlying systemic diseases favoring the development of these infections. Illnesses such as diabetes or cancer have been described in over 90% of cases of progressive bacterial gangrene.
Of patients with necrotizing fasciitis, 20-40% are diabetic. As many as 80% of Fournier gangrene cases occur in people with diabetes. In some series, as many as 35% of patients were alcoholics. However, approximately one half of the cases of streptococcal necrotizing fasciitis occur in young and previously healthy people.
A study by Hung et al suggested that liver cirrhosis is an independent risk factor for necrotizing fasciitis. In a retrospective analysis of hospital data, the investigators determined the incidence of necrotizing fasciitis development in 40,802 patients with cirrhosis and 40,865 control patients, over a three-year follow-up period after each patient’s initial hospitalization. Necrotizing fasciitis occurred during follow-up in 299 patients with cirrhosis (0.7%) and in 160 control patients (0.4%), giving patients with cirrhosis a hazard ratio of 1.98 for necrotizing fasciitis. It was also found that the risk of necrotizing fasciitis was greater in patients with complicated cirrhosis than in those with the noncomplicated type (hazard ratio 1.32). [44]
Studies have shown a possible relationship between the use of nonsteroidal anti-inflammatory agents (NSAIDs), such as ibuprofen, and the development of necrotizing fasciitis during varicella infections. Additional studies are needed to establish whether ibuprofen use has a causal role in the development of necrotizing fasciitis and its complications during varicella infections. This has not previously been described.
Group A beta-hemolytic streptococci have historically been noted as a cause of necrotizing fasciitis, but Haemophilus aphrophilus and S aureus are also associated with the condition, and some patients have mixed infections involving multiple species of bacteria, including mycobacteria, as well as fungi. [45, 46]
A synergistic infection with a facultative anaerobic bacterium may be significant. In 1 patient, Phycomycetes appeared to be responsible for necrotizing fasciitis.
Streptococcus pneumoniae is a rare cause of necrotizing fasciitis. [45] In one patient, S pneumoniae serotype 5 was also isolated. This serotype 5 antigen is included in the polysaccharide 23-valent pneumococcal vaccine, highlighting the value of pneumococcal immunization.
In type I necrotizing fasciitis, anaerobic and facultative bacteria work synergistically to cause what may initially be mistaken for a simple wound cellulitis. A variant of type I necrotizing fasciitis is saltwater necrotizing fasciitis in which an apparently minor skin wound is contaminated with saltwater containing a Vibrio species.
In type II necrotizing fasciitis, varicella infection and the use of nonsteroidal anti-inflammatory drugs may be predisposing factors.
Type III necrotizing fasciitis is usually caused by Clostridium perfringens. When type III necrotizing fasciitis occurs spontaneously, C septicum is more likely to be the etiologic agent; these cases usually occur in association with colon cancer or leukemia.
Unusual causes include injection anthrax. [47] Rapidly progressive necrotizing fasciitis following a stonefish sting has been described in 2 patients. [48]
Prognosis
The reported mortality in patients with necrotizing fasciitis has ranged from 20% to as high as 80%. [49, 36, 38, 50] Pathogens, patient characteristics, infection site, and speed of treatment are among the variables that affect survival.
Poor prognosis in necrotizing fasciitis has been linked to infection with certain streptococcal strains. However, McHenry et al found that monomicrobial infection with S pyogenes was not associated with an increased mortality. [38]
A retrospective study by Hsiao et al found that Aeromonas infection, Vibrio infection, cancer, hypotension, and band form WBC count greater than 10% were independent positive predictors of mortality in patients with necrotizing fasciitis, while streptococcal and staphylococcal infections were not identified as predictors of mortality. Hemorrhagic bullae appeared to be an independent negative predictor of mortality. However, accuracy of these factors needs to be verified. [51]
A retrospective cohort study by Chang et al of patients with necrotizing fasciitis who underwent amputation reported that in those individuals in whom amputation was performed more than 3 days after admission, the mortality risk was higher when hemorrhagic bullae, peripheral vascular disease, or bacteremia was present or the laboratory risk indicator for necrotizing fasciitis (LRINEC) score was over 8. The investigators recommended that in cases in which any of these risk factors is present, amputation not be delayed more than 3 days postadmission. [52]
In another study, preexisting chronic liver dysfunction, chronic renal failure, thrombocytopenia, hypoalbuminemia, and postoperative dependence on mechanical ventilation represented poor prognostic factors in monomicrobial necrotizing fasciitis. In addition, patients with gram-negative monobacterial necrotizing fasciitis had more fulminant sepsis. [53]
Similarly, a retrospective study by Adachi et al reported that in patients with necrotizing fasciitis, renal dysfunction predicts fatal outcomes. Estimated glomerular filtration rate was a prognostic factor, the cutoff value being 20.6 mL/min. [54]
The mean age of survivors is 35 years. The mean age of nonsurvivors is 49 years.
A retrospective review by Cheng et al showed that upper extremity necrotizing fasciitis has a high mortality rate. In their review, about 35% of patients died. A state of altered consciousness and respiratory distress at initial presentation were found to be statistically significant factors for eventual mortality. Early diagnosis and referral for aggressive surgical treatment prior to the development of systemic toxic signs are essential for survival. [55]
In a retrospective review of craniocervical necrotizing fasciitis, Mao et al reported a survival rate of 60% for patients with thoracic extension (6 of 10) compared with 100% for those without thoracic extension. Lower overall survival for the patients in the thoracic extension group was attributed to older patient age, greater comorbidity, need for more extensive surgical debridement, and increased postoperative complications.
Better survival of the patients without thoracic extension was attributed to aggressive wound care and surgical debridement, broad-spectrum intravenous antibiotics, and care in the surgical intensive care unit. [56]
A study by Friederichs et al indicated that necrotizing fasciitis tends to have a worse outcome when acquired iatrogenically via injection or infiltration than it does when acquired in other ways, with higher mortality and amputation rates (67% and 73%, respectively) found. The study included 21 patients with injection- or infiltration-related necrotizing fasciitis and 134 patients who were infected with the condition through other means. [57]
In a study by Rouse et al, the overall mortality rate was 73% (20 of 27 patients). They indicated that prompt recognition and treatment of necrotizing fasciitis was essential: Of 12 patients whose treatment was delayed for more than 12 hours, 11 patients died. [36]
Similarly, McHenry et al reported that the average time from admission to operation was 90 hours in nonsurvivors of necrotizing soft-tissue infections; in survivors, this average time was 25 hours. [38] Early debridement of the infection was obviously associated with a significant decrease in mortality.
Necrotizing fasciitis survivors may have a shorter life span than population controls, owing to infectious causes such as pneumonia, cholecystitis, urinary tract infections, and sepsis. [58]
Patient Education
Necrotizing fasciitis has become sufficiently common in the United States that a National Necrotizing Fasciitis Foundation has been formed. The cofounders of this foundation are survivors of necrotizing fasciitis. The goals of this foundation are to increase awareness about necrotizing fasciitis, to educate the medical community, and to help save lives from this often misdiagnosed, rapidly fatal disease.
The foundation’s website has stories about survivors of necrotizing fasciitis with color photographs. The Foundation provides a list of journal articles as well as 8 brief reports on necrotizing fasciitis that can be downloaded at no cost.
-
Left upper extremity shows necrotizing fasciitis in an individual who used illicit drugs. Cultures grew Streptococcus milleri and anaerobes (Prevotella species). Patient would grease, or lick, the needle before injection.
-
Necrotizing fasciitis at a possible site of insulin injection in the left upper part of the thigh in a 50-year-old obese woman with diabetes.
-
Photomicrograph of Fournier gangrene (necrotizing fasciitis), oil immersion at 1000X magnification. Note the acute inflammatory cells in the necrotic tissue. Bacteria are located in the haziness of their cytoplasm. Courtesy of Billie Fife, MD, and Thomas A. Santora, MD.
-
Left lower extremity in a 56-year-old patient with alcoholism who was found comatose after binge drinking. Surgical drainage was performed to treat the pyomyositis-related, large, non–foul-smelling (sweetish) bullae. Gram staining showed the presence of gram-positive rods. Cultures revealed Clostridium perfringens. The diagnosis was clostridial myonecrosis.
-
Sixty-year-old woman who had undergone postvaginal hysterectomy and repair of a rectal prolapse has a massive perineal ulceration with foul-smelling discharge. Cultures revealed Escherichia coli and Bacteroides fragilis. The diagnosis was perineal gangrene.
-
Necrotizing fascitis of entire thoracolumbar posterior area in 20-year-old patient with chronic myelogenous leukemia and neutropenia (WBC count, 680/uL). Cultures revealed gram-negative Pseudomonas species and Bacteroides fragilis.









